Data-Driven Process Improvement Raises Patient Safety for Highest-Risk Medication
“Improvement is about getting the necessary stakeholders involved in the process as it’s developed, not just at the end of the road when you have a product ready to present. That way, when you are ready to present final protocols for approval, they already know about it, they understand it, and the actual approval is a non-event.”
– David Beddow, MD
Medical Director, Hospitalist Services, Allina Health System
- A seven percent relative improvement in the percentage of patients therapeutic within 24 hours of protocol initiation.
- Paring 20+ site-based documents (e.g., policies, protocols, and order sets) to one systemwide guideline and four systemwide protocols.
Intravenous (IV) heparin is widely used1,2 to prevent thrombosis in a variety of clinical settings, yet it is considered one of the highest-risk medications3,4 used in the inpatient setting because of the potential for dosing errors. Allina Health identified multiple IV heparin protocols among its hospitals, a variation that increased the risk of errors. Standard practices that addressed patients’ clinical needs in a disease-specific way were lacking. Over the course of 1.5 years, more than 9,000 patients at Allina Health had an IV heparin protocol ordered, so IV heparin safety was of utmost concern.
To address this quality issue and improve clinical value, Allina Health created a systemwide interdisciplinary team to standardize IV heparin therapeutic guidelines and monitor the impact of the standard guideline on patient outcomes. Allina Health engaged multiple physician stakeholder groups to review proposed protocols and provide critical feedback to help ensure the best possible patient care and safety. To effectively monitor IV heparin outcomes, patient safety, and the impact of the new, standard guidelines and protocols, Allina Health developed an anticoagulation safety analytics application, using the Health Catalyst Analytics Platform, including the Late-Binding™ Data Warehouse and broad suite of analytics applications.
IV Heparin: A High-Value, High-Risk Medication
Intravenous (IV) heparin provides many clinical benefits, but is a medication with significant risks because of the potential for injury. Sub-therapeutic levels can lead to embolism or recurrent deep vein thrombosis, while supra-therapeutic levels can lead to major bleeding complications.
Monitoring the therapeutic levels of heparin is critically important. While standardized dosing guidelines exist, each patient clears heparin at a slightly different rate, making dose response somewhat unpredictable. Heparin levels are measured by lab tests that check the concentration of heparin in the blood. There are two tests available: activated partial thromboplastic time (aPTT) and antifactor Xa assay (anti-Xa).
Allina Health, an integrated delivery system throughout Minnesota and western Wisconsin, includes 12 hospitals with more than 109,000 inpatient admissions annually, and more than 1.45 million hospital outpatient admissions. Over the course of 1.5 years, more than 9,000 patients at Allina Health had an IV heparin protocol ordered.
Variation Increases Risk of Harm from High-Risk Medication
Allina Health identified multiple IV heparin protocols among its hospitals, some with more than one treatment protocol. Standard practices that addressed patients’ clinical needs and met regulatory guidelines did not exist, and opinions regarding how to best manage, administer, and monitor IV heparin varied among the numerous specialty groups.
Variation in protocols substantially increases the risk of dosing errors. Effective titration of IV heparin typically requires administration of an initial loading bolus, a titrated weight-based drip rate, and repeat bolus doses. The titrated drip rate for IV heparin and subsequent bolus doses are based on the lab results from the anti-Xa (or aPTT) test. Allina Health was aware that appropriate dosing was already a complicated process that needed to be performed consistently to ensure safety. Unnecessary, unintended variation in protocols increased the potential for error of an incredibly high-risk medication.
Allina Health was also largely unable to monitor the impact of the various treatment protocols on patient outcomes. The only ways to collect data for the various treatment protocols were to use reports of adverse outcomes or conduct burdensome manual chart reviews, which could take weeks or months to complete. Limited resources to perform the manual chart review, and the lag time between identifying a potential problem and completing the manual chart review, did not necessarily produce meaningful data that could be used for timely process or patient safety improvement.
To assure patient safety, Allina Health needed to eliminate unnecessary variation in IV heparin administration and monitoring, and needed a solution that would enable it to efficiently and effectively monitor the impact of the treatment protocol on patient outcomes.
Analytics Supports Evidence-Based Decision Making and Improves Patient Safety
Improvement Teams and Stakeholder Support Improve Processes of Care
Allina Health’s Improving Clinical Value Program engages employees and physicians in decreasing clinical variation for improved outcomes and reducing unnecessary costs to patients, communities, and Allina Health. Improving Clinical Value aligns the entire organization behind clinically driven work to achieve the best outcomes and care for patients, requires a multi-disciplinary team across the entire system, including physicians, bedside nurses, pharmacy, supply chain, finance and clinical analytics. To achieve the desired outcomes and create value that can be differentiated, Improving Clinical Value requires support from hospitals, clinics, clinical service lines, home care services, network integration and system office. The Improving Clinical Value Program reliably supports leaders in identifying opportunities for improvement that positively impact the patient and the organization, and includes a process to assess the value of these improvements prior to initiating them.
As part of its Improving Clinical Value Program, Allina Health commissioned, and made resources available to support, an IV heparin project to create a systemwide interdisciplinary team that comprised providers, pharmacists, data analysts, operational leaders, registered nurses, finance, project management, and EHR experts. The team had two goals:
- Standardize IV heparin therapeutic guidelines.
- Establish a mechanism to monitor the standard guideline’s impact on patient outcomes.
The heparin improvement team followed the Allina Health model for evidence-based decision making to ensure the incremental development of the systemwide guidelines and protocols, engage stakeholders throughout the process, and ensure appropriate communication, education, and support of guideline adoption.
For IV heparin, multiple physician stakeholder groups, including vascular medicine, critical care, hospitalists, neurocritical care, and emergency physicians, were engaged to review proposed protocols and provide critical feedback to help ensure the best possible patient care and safety.
To establish the standard guidelines, the team reviewed the literature and critiqued the evidence, and ultimately decided to use aPTT as the standard lab for monitoring therapeutic levels. The team reviewed guidelines published within the past five years, and literature published within the past 10 years that focused on adult inpatients. It compared the use of anti-Xa and aPTT in monitoring unfractionated heparin protocols and evaluating safety, efficacy, and cost. The team identified no demonstrable difference in patient outcomes with the use of anti-Xa, and selected the lower-cost aPTT as the Allina Health standard.
Sub-teams evaluated available literature to develop a systemwide guideline and indication-specific IV heparin protocols for venous thromboembolism (VTE) prophylaxis, high bleed risk patients, acute coronary syndrome, and atrial fibrillation.
Pharmacist expertise, input, and guidance were critical to develop and adopt the system guideline and indication-specific protocols, provide guidance, education and support, and actively monitor patient lab results during the implementation feedback, supporting the registered nurses in making appropriate infusion rate adjustments.
Using Analytics to Ensure Patient Safety
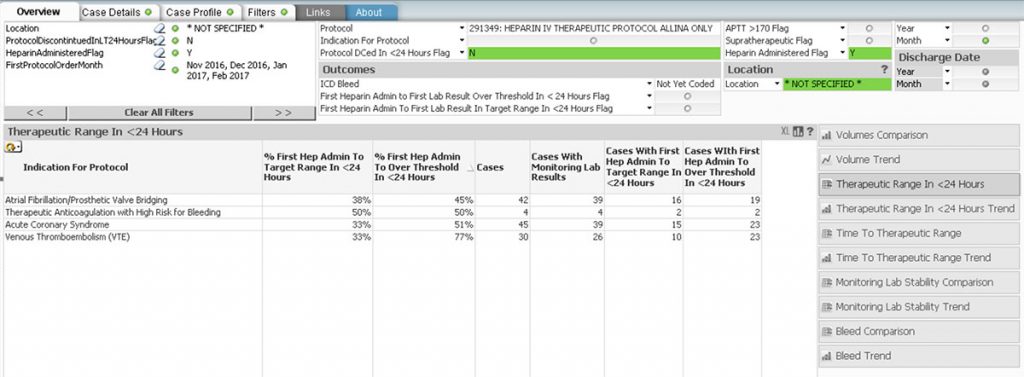
To effectively monitor IV heparin outcomes, patient safety, and the impact of the new, standard guidelines and protocols, Allina Health used the Health Catalyst Analytics Platform, including the Late-Binding™ Data Warehouse and broad suite of analytics applications, to develop an anticoagulation safety analytics application (see Figure 1).
The anticoagulation safety application supported the heparin improvement team’s review of a variety of data:
- Indications for the protocol.
- Percentage of patients to therapeutic target range within 24 hours by protocol type.
- Adverse outcomes, such as bleeding complications.
- List of all patients receiving IV heparin.
The anticoagulation safety analytics application also supported the heparin improvement team with targeted case reviews. The application’s Case Details tab included a summary of the case, all ordered protocols, lab reports, recording of medications administered, diagnosis, procedures, imaging, and patient outcomes. This allowed the improvement team to review pertinent aspects of the patient’s care without having to log on to and search the EHR.
Results
Previously, it took weeks, or months, to conduct the manual chart review required to evaluate protocol effectiveness. With the anticoagulation safety analytics application, the heparin improvement team can monitor protocol effectiveness and the impact on patient safety in just a few hours. The team continues to review process measures and clinical outcomes for each indication-specific protocol at least every eight weeks.
Additionally, the team successfully improved clinical value through processes of care and clinical outcomes associated with IV heparin administration. Results include:
- Seven percent relative improvement in the percentage of VTE patients therapeutic within 24 hours of protocol initiation.
- Reduction from 20+ site-based documents (e.g., policies, protocols, order sets) to one systemwide guideline and four indication-specific protocols.
What’s Next
Allina Health plans to continue using the anticoagulation safety analytics application to evaluate the effectiveness of the indication-specific protocols and assure safety for patients receiving IV heparin. Having successfully standardized IV heparin therapeutic guidelines, and developed and validated the anticoagulation safety dashboard to monitor the standard guideline’s impact on patient outcomes, the heparin team will conclude its work and handoff future monitoring to the larger pharmacy and therapeutics teams.
References
- Patient Safety Authority, Pennsylvania Patient Safety Advisory. (2006). Let’s stop the bleeding: Preventing errors with heparin therapy, 3(4):31.
- Crowther, M. A., & Warkentin, T. E. (2008). Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: Focus on new anticoagulant agents. Blood, 111(10), 4871-4879.
- Agency for Healthcare and Quality. (2015). Medication Errors.
- Vanderveen, T. (2009). Vial mistakes involving heparin. Agency for Healthcare Research and Quality, Patient Safety Network.
This website stores data such as cookies to enable essential site functionality, as well as marketing, personalization, and analytics. By remaining on this website you indicate your consent. For more information please visit our Privacy Policy.