Allina Health created a framework for standardizing evidence-based practices in treatment to reduce variation and waste. This involved collaboration among stakeholders and establishing guidelines for Stage 1 lung cancer and IV heparin. A comprehensive checklist ensures adherence to guidelines, leading to improved outcomes and reduced costs.
To tackle the variation and waste that can arise from different treatment decisions, Allina Health developed a solid framework to establish and deploy standard, evidence-based practices across the enterprise. The transition to a standard evidence-based decision-making process required collaboration and buy-in from multiple stakeholders and physicians. Allina’s established quality governance structure reviewed and approved systemwide clinical practice guidelines for Stage 1 lung cancer treatment and IV heparin treatment.
To sustain and improve on this new model of care, a comprehensive checklist was developed to ensure that all future guidelines are based on patient subgroups and preferences, available evidence, stakeholder review, and other important criteria including IOM standards. Adherence to guidelines is monitored with metrics based on data extracted from Allina’s enterprise data warehouse and from the electronic health record.
Results to date already indicate notable improvements in variation and cost.
Evidence has always played a key role in healthcare, and there is no shortage of clinical studies and published research to consult. The very proliferation of this information, however, makes it almost impossible to keep up with the most current findings.1 To that end, clinicians continue to have to make difficult decisions on a regular basis, often with a good deal of uncertainty.2 To reduce the significant variance and waste that arise when different clinicians within the same health system make markedly different decisions about medical treatment,3 health systems like Allina Health are taking the lead on implementing evidence-based decision-making models and clinical practice guidelines (CPGs).
In essence, this is the framework for improving outcomes based on the best possible evidence. It also supports shared decision making between patients and their providers (see Figure 1).

The following story describes how Allina accomplished significant improvements across a vast enterprise of 13 hospitals, over 90 clinics, 16 pharmacies and numerous specialty medical practices—and further cemented its brand promise that all patients will receive optimal care regardless of treatment location.
As a not-for-profit health care system dedicated to helping people live healthier lives in communities throughout Minnesota and western Wisconsin, Allina has a wide regional reach. While this assures quality care for more patients, deploying evidence-based practices across such a large system comes with unique challenges. The processes for developing clinical practice guidelines at Allina, for example, were historically isolated within different units or locations. Additionally, there was no systemwide policy or infrastructure in place to devise and establish these guidelines. This made it difficult to deploy and update the guidelines that did exist across the entire enterprise.
All of this was compounded by another common challenge—Allina’s busy physicians did not have the resources and assistance needed to regularly review the latest research literature.
The sum challenge was two-fold. First, Allina clinicians needed the right clinical practice guidelines in hand to reduce variation and make sure that all patients, regardless of location, received the best possible care. Just as importantly, these guidelines would need to be part of a larger, evidence-based decision-making model in which administrators and other stakeholders could measure compliance with evidence-based practices, and have access to data that would help identify opportunities for improvement.
The work of crafting sound clinical practice guidelines began with the development and testing of a systemwide, measurable EBDM model (see Figure 2). This model provided a standard, step-by-step blueprint for guideline development. Allina has dedicated resources to collect and assess available evidence for selected clinical conditions, making it easy for participating physicians to review the evidence rather than spending their valuable time searching the literature.
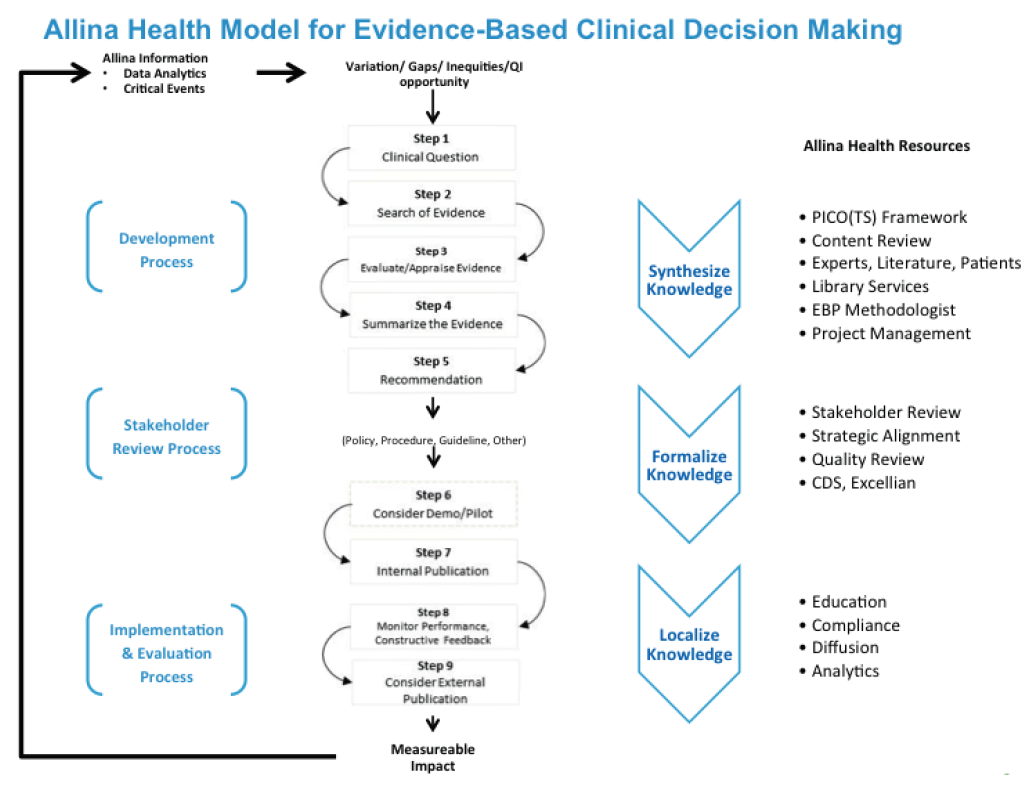
Allina recognized that a systemwide policy on guidelines would support and uphold the EBDM model. Accordingly, this policy was comprised of several key elements. First, it includes a process for peer review and approval of proposed guidelines and a central repository to access approved guidelines. In addition, Allina established a multidisciplinary physician-led Clinical Practice Council to prioritize, develop, and make peer-reviewed, evidence-based recommendations for specific systemwide issues such as opioids for acute pain, medical cannabis, and appropriate age for mammography screening. Allina’s senior leadership made their support visible throughout the entire process.
Initial focus was on clinical areas that represented significant variation in practice and/or where there was a lack of consensus. High motivation and readiness among providers to adopt evidence-based clinical practice guidelines prioritized initial phases testing the EBDM model. Two areas in particular were identified for initial improvement:
A thorough checklist was created for guideline development, implementation, and adherence. First, guidelines are to be developed by knowledgeable, multidisciplinary panels of experts and representatives from key affected groups. From there, they must adhere to certain important criteria.
Working together, healthcare providers, knowledge acquisition experts, program managers, and others helped move the project efficiently from development to full implementation—and then to even further scaling out. Allina was able to overcome clinical skepticism through a combination of engaged physician leadership, education, collaborative guideline development, policies, and effective communication. Hiring a dedicated resource who could own this work was also vital to clinician engagement as well as the overall success of this initiative.
With the EBDM model in place, Allina will leverage its deep partnership with Health Catalyst to extract near real-time data in the analysis of compliance and the impact these guidelines are having on outcomes.
Allina has created and adopted an effective, systemwide EBDM model that facilitates collaborative guideline creation. The success here rests on the collaborative approach Allina took to developing the model, backed it up with a policy, and implemented across the health system over a 9-month period. Indeed, the EBDM initiative reinforced systemwide collaboration and partnerships across Allina. Preliminary results from testing of the model indicate high stakeholder buy-in, utility, and feasibility within the current system.
Allina has also observed an overall increase in understanding of, and appreciation for, systematic approaches to EBDM. As for the costs of implementing the EBDM model, beyond one incremental full-time employee and portions of people’s time, these costs have been minimal.
“The process of developing clinical practice guidelines has become both efficient and collegial using the evidence-based decision-making model. It is a highlight of my career.”
– Lee Kamman, MD, Pulmonologist, Allina Health
Allina is continuing to spread the guideline development policy and EBDM model to additional clinical areas and conditions. Allina also continues to develop and implement metrics and acquire data to measure compliance, drive accountability, and document improvement in clinical outcomes and costs.


