With a potential industry-wide savings of almost $21 billion and an impact on more than seven million patient lives, preventing harmful medication error is a significant improvement opportunity for health systems. Also known as adverse drugs events (ADEs), harmful medication errors comprise about 37 percent of all medical harm. Approximately 50 percent of ADEs are preventable, making their reduction a highly impactable area of patient safety.
Current data and analytics workflow tools are making ADE surveillance, monitoring, and prevention increasingly more effective with four key capabilities:
1. Perspective surveillance for ADEs and identification of previously undescribed ADEs.
2. Identification of the root cause of many ADEs by drug class.
3. Prescription at appropriate doses for patients with compromised kidney or liver functions.
4. Identification of different types of harm to find causes.
 Download
Download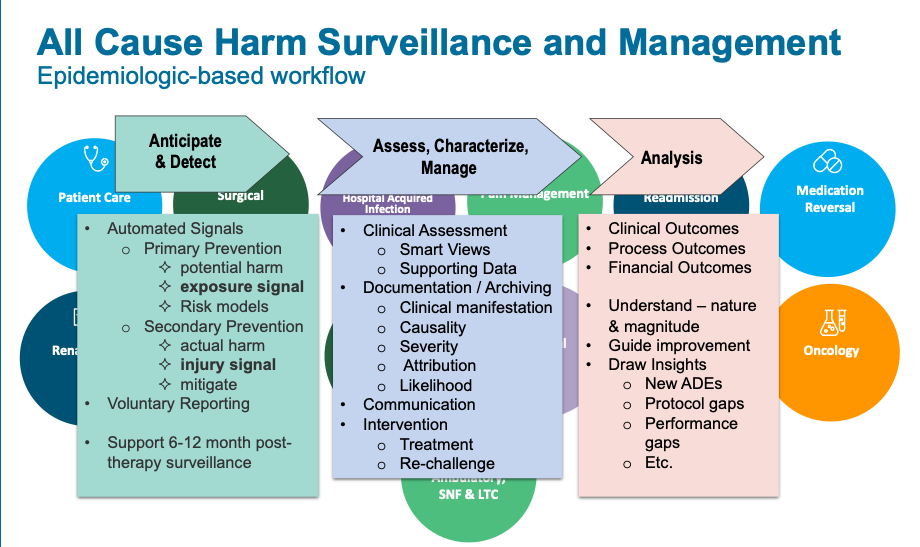

With the majority of hospitalized patients today receiving medications, health systems face a significant risk for harmful medication errors, or preventable adverse drug events (ADEs). According to a 2008 estimate from the U.S. Department of Health and Human Services, patient harm due to ADEs comprise a sizeable yet impactable portion of safety events:
Error (medication of other) doesn’t correlate one-to-one with harm. Errors can be near misses, in which a mistake or miscalculation doesn’t make it to the patient; only a small portion filter through to cause harm.
The breadth and depth of ADE impact creates an obvious cost and quality issue for healthcare organizations but also a sizeable opportunity for improvement: an almost $21 billion in potential savings and an impact on more than seven million patients. Health systems haven’t historically addressed ADEs effectively. Today, however, with decision support software enabling real-time surveillance of patient populations, healthcare stands to make significant improvements in ADE detection, monitoring, and prevention.
Shared interest in drug safety between the FDA and health systems sparked initial research into ways to identify ADEs. In the 1990s, a large health system used its EMR data to identify specific types of patient harm, similarly to how it used the EMR in infectious disease surveillance. The health system started using its EMR data to develop triggers for specific types of patient harm (e.g., sudden medication-stop orders, antidote ordering, and certain abnormal laboratory values). The trigger system generated a daily list of potential ADEs among the patient population, and pharmacists classified risk according to severity and risk type (e.g., dose dependent, predictable, idiosyncratic, or allergic).
Using EMR data and triggers over 18 months, the health system verified 731 ADEs in 648 patients. They compared the data– and analytics-enabled approach to traditional detection methods, which identified only nine ADEs during the same period.
Drug safety remains a hot topic in healthcare, particularly as leading-edge therapies come to market after clinical trials that include only hundreds or thousands of patients from a limited demographic. For example, when the FDA approves a new chemical entity for immunotherapy for cancer treatment available, the drug may have appeared relatively safe in clinical trial participants. Pre-marketing clinical trial enrollment criteria, however, tends to exclude patients with significant comorbidities (e.g., cardiovascular or renal disease).
When the drug reaches the general public, prescribing clinicians don’t have the data or clinical trial experience to know how it affects people with a variety of comorbidities. These blind spots, along with exposing large groups of patients to the agent (often larger numbers than in the original clinical trials), create a risk for ADEs that the premarketing, phase three trials didn’t discover. If the general population outcomes are bad, the FDA removes the drug from the market. Pharmaceutical companies stand to lose up to millions of dollars invested in getting the drug to market, and patients lose the opportunity for a potentially effective therapy.
This gap in the drug approval process—from approval to the general public—opens the opportunity for data- and analytics-enabled post-marketing surveillance. A focused surveillance system for new drugs and devices that provides rigorous, prospective post-marketing surveillance could enable early release of new therapies. The FDA could use this period to get real-time, broader-population understanding of the ADE risks and adjust guidelines accordingly. Extended surveillance could mean that more drugs make it to the general public with greater safety understanding. Theoretically, fewer potentially promising therapies would be removed from the market due to newly discovered serious or life-threatening ADEs.
A clinical workflow (decision-support) tool that leverages triggers (e.g., the Health Catalyst® Patient Safety Monitor™ Suite: Surveillance Module) allows drug safety teams to evaluate and take critical action against ADEs:
For example, using decision-support triggers, the workflow tool focuses clinician attention on a patient receiving an anticoagulant who has a lab result for unexplained bleeding—an activated partial thromboplastin time (aPTT) of greater than 300 and a systolic blood pressure of <90 mmHg. This indicates that the patient may be experiencing a major drug-induced bleeding event. The workflow tool guides the clinician to drugs that may be causing this serious adverse event and offers further guidance on how to mitigate the event. Advanced analytics derived from automated, prospective surveillance—combined with clinical evaluation that includes structured root cause analysis, likelihood, and severity assessment—provide epidemiologically sound data for machine learning algorithms. These algorithms drive advanced decision support applications designed to prevent adverse events in future patients treated with the offending agent or similar agents.
Figure 1 shows the ADE clinical surveillance workflow, including detection; characterization and analysis; and measurement. The workflow gives clinicians the data they need to understand and make critical decisions about ADE mitigation:

Post-marketing drug surveillance with an ADE clinical surveillance workflow tool helps improve patient safety for hospitalized patients in four key ways:
As healthcare continues to develop and use more, increasingly powerful drugs, risks for ADEs will rise along with the potential for effective treatments. One way that health systems can help manage patient harm due to medication is to adopt a decision support tool that enables real-time surveillance of patient populations and advanced analytics to identify risk for ADEs and suggest preventive action.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download the presentation highlighting the key main points.
Click Here to Download the Slides
https://www.slideshare.net/slideshow/embed_code/key/t556JLaGI7h0Oi